Solution:
We know that:
- The half-life of Iodine-131 is 8.065 days
- From Nov. 3 to Nov. 11th is 8 days
- From Nov. 11th 1200 h to Nov. 27th at 0800h is (16 days less 4 hours) =15.833 days
- Let A0 denote the (unknown) initial acitivity on
delivery day, Nov 3rd, at 1200 h. Since it is known that 8 days
later the activity is 370 MBq:
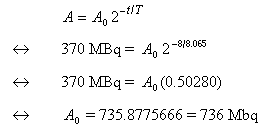
Answer: The activity on delivery day, Nov 3rd, at 1200 h, is 736 MBq.
- For this part, consider the activity 370 MBq
at 1200 hours on Nov. 11th to be the initial activity,
A0. Let the unknown
activity 15.833 days later (on Nov. 27th at 0800 h) be
A:
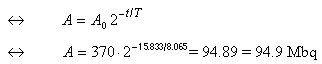
Answer: The activity on Nov. 27th at 0800 h, is 94.9 MBq.