Solution:
Newton’s Law of Cooling states:
Where T is the temperature of a cooling object at time t , T 0 is the temperature of the object at time t = 0, T s is the temperature of the surrounding medium, and k is the decay constant.
For this problem, ![]()
![]() (1)
(1)
The first thing we need to do is find k .
Substituting T = 185° F, when t = 5.0 minutes, into (1), we get:
And, simplifying, we get:
Taking natural logs of either side, we get: ln(125) - ln(165) = -5.0 k
And ![]()
![]() (2)
(2)
-
To find how long it will take for T to reach 120° F, we substitute into (2) and solve for t
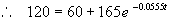 , and simplifying, we get:
, and simplifying, we get:
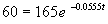
Taking natural logs of either side, we get: ln(60) - ln(165) = -0.0555 t
And
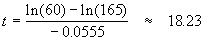
∴ it will take about 18.0 minutes for the solution to cool down to 120° F
-
To find the temperature of the solution after t = 30 minutes, we substitute again into (2)
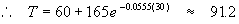
So we find that after 30 minutes, the temperature of the solution will be about 91° F.