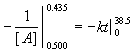The problem is to find the rate of change of the reactant in
a 2nd order chemical reaction which can be modelled
by the given by the differential equation, 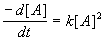 ,
where [A] is the concentration of the reactant at time
t in minutes, and k is the reaction constant.
,
where [A] is the concentration of the reactant at time
t in minutes, and k is the reaction constant.
If [ A ] = 0.500 M when t = 0 minutes, and [ A ] = 0.435 M when t = 38.5 minutes, find k .